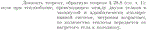Task id16884
Positive responses: 0
Negative responses: 0
Sold: 0
Refunds: 0
$0.22
Prove the theorem converse to theorem § 28.8 (see Vol. 1): if during heat exchange occurring between two bodies in a closed and adiabatically isolated system, entropy increases, then the amount of heat is transferred from the heated body to the cold one.
Detailed solution. Format gif
No feedback yet