Task id16326
Positive responses: 0
Negative responses: 0
Sold: 1
Refunds: 0
Sorry, but this item is temporarily out
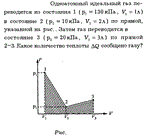
A monatomic ideal gas is transferred from state 1 (p1 = 130 kPa, V1 = 1l) to state 2 (P2 = 10 kPa, V2 = 2l) along the straight line shown in the figure. Then the gas is transferred to state 3 (P3 = 20 kPa, V3 = 3l) in a straight line 2-3. What amount of heat dQ is imparted to the gas?
Detailed solution. Format gif
No feedback yet